Lewis Structure - The Lewis Structure (Electron-Dot) of a molecule or polyatomic ion shows how the valence electrons are arranged among the atoms in the molecule or ion. The rule to build the Lewis Structure is based on chemists' experience: The most important requirement for the formation of a stable compound is that atoms achieve noble gas electron configurations by sharing electrons with other atoms, i.e. satisfied the Octet Rule. It is also called Lewis Dot Symbol, which consists of the symbol of an element and one dot for each valence electron in an atom of the element. In this model, the valence of an element is the number of electron pairs shared to complete the octet rule. The covalent bond between two atoms is formed by sharing the valence electrons.
Resonance Structures are two or more Lewis Structures for a molecule or ion that have the same arrangement of atoms, contain the same number of electrons, and differ only in the location of the electrons. For some molecules, the resonance structures are needed to correctly describe the electronic structure of the molecule.
This module is to compute the number of valance electrons in a molecule and construct a Lewis structure by combining electron pairs according to the Octet rule. The resonance structures are also displayed if existed.
The step-by-step illustrations and instructions will guide you through the solution of the problem you input. Click Next Step to go forward and click Previous Step to go back.
1. Click Lewis tab to open Lewis Structure module. The Lewis Structure module appears.
On the left pane, it is the Compute module and one the right is the Guide page. The top pane is the navigational toolbar.
2. Enter the formula of a molecule from Input field - Enter a Molecular Formula, in this case, NO3-.
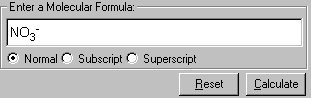
Note: To enter subscript or superscript, use the Subscript or Superscript options below the input box. Alternatively, you can use <Up Arrow> to enter superscript or <Down Arrow> to enter subscript. The keyboard sequence to enter NO3- is N-O-<Down Arrow>-3-<Up Arrow>-"-".
3. Click Calculate to proceed. If you need to clear the input, click Reset.
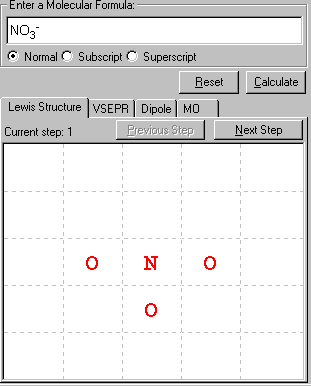
4. The step-by-step solution and guide will follow. Click Next Step to go forward and click Previous Step to go back.
This is to determine the arrangement of the atoms in the molecule or polyatomic ion. First the central atom is determined, in this case N, then the ligand atoms are defined, three O atoms.
Note: If the atomic arrangement above is incorrect, you can switch the positions of any two atoms by dragging the first atom (click on the 1st atom and hold/move the left-mouse button) and drop (mouse over the 2nd atom and release the left-mouse button) onto the second atom. The positions of these two atoms will be swapped.
This step is to determine the total number of valence electrons present, that is, the total number of dots that must appear in the electron-dot structure. To do so, first determine the valence electrons of each atom. The valence electrons are represented by dots around the atomic symbols. The total number of valence electrons in a molecule is the sum of each contributor.
The program counts the number of valence electrons in this molecule, if this is +1 cation, subtract one electron; if -1 anion, add one electron. In this case , the total valence electrons = 5 (N) + 3x6(O) + 1 (anion) = 24, which is displayed on the bottom of the screen.
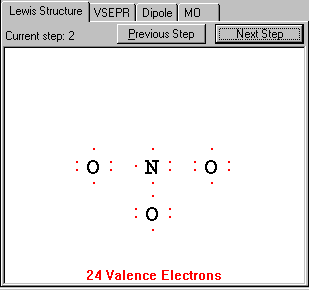
Draw a single bond between each pair of atoms bonded together. Do not worry about the electrons and multiple bonds at this step. Just connect atoms together with single bonds.
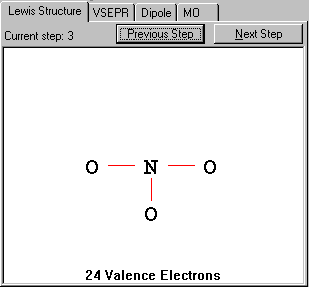
In this case, there are three single bonds needed to connect all three O atoms to the central atom N.
Complete the octets of the ligand (surrounding) atoms first. Hydrogen atoms need only two electrons.
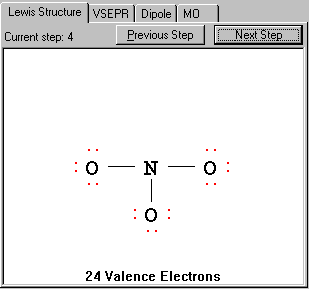
In this case, three N-O bonds takes six electrons and 3x6=18 electrons needed to fulfill the octet for the three surrounding O atoms, totalling 24 electrons. No more electrons left for the central atom N. The program will skip this step.
If there are electrons left, they will be placed on the central atoms.
If there are not enough electrons to give the central atom an octet, try multiple bonds. Use one or more of the unshared pairs of electrons on the atoms bonded to the central atom to for double or triple bonds. In this example, after connecting the central atom with ligand atoms and filling electrons onto the ligand atoms by Octet Rule, there is no more electrons left.
If the central atom is not satisfied with the Octet Rule (that is the case here, N has only 6 electrons), a multiple bond might be needed by using both electrons from the outer atom (e.g. O atom).
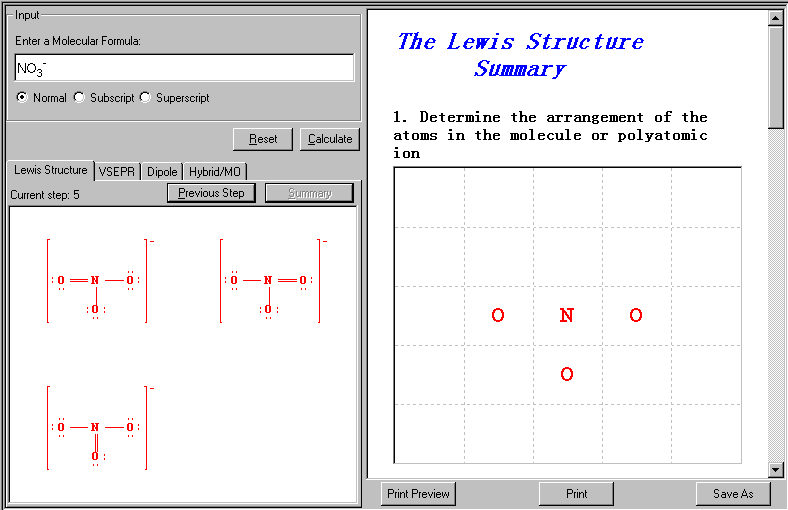
Resonance Structure is one of two or more Lewis structures for a single molecule that can not be described fully with only one Lewis structure. In some cases, resonance structures, using two or more Lewis structures, may be used to represent a particular molecule. In this case, the following three Lewis structures all satisfy the Octet rule, together, they present the overall molecular structure of the compound.

In this example, since all N-O bonds are equivalent (with the same bond length), equivalent Lewis structures can be drawn as shown above.
That is the end of the walkthrough for generating a Lewis Structure for a given formula.