MO Theory- Molecular Orbital Theory describes covalent bonds in terms of molecular orbitals, which result from interaction of the atomic orbitals of the bonding atoms and are associated with the entire molecule. A bonding molecular orbital has lower energy and greater stability than the atomic orbitals from which it was formed. An antibonding molecular orbital has higher energy and lower stability than the atomic orbitals from which it was formed. Placing electrons in a bonding orbital yields a stable covalent bond.
This module is to calculate and display the MO energy diagram of diatomic molecules (both homonuclear and heteronuclear) and show the hybrid orbitals of polyatomic molecules.
This is an one-step solution. In the case of diatomic molecules, the MO diagram with filled electron is shown,. For polyatomic molecules (more than two atoms in a molecule), the hybrid orbitals of the central atom is displayed.
1. Click Hybrid/MO tab to open Hydrid/MO module.
On the left pane, it is the Compute module and one the right it is the Guide page. The top pane is the navigational toolbar.
2. Enter the formula of a molecule from Input field - Enter a Molecular Formula, in this case, He2.
2. Enter the formula of a molecule from Input field - Molecular Formula.
3. Click Calculate to proceed. If you need to clear the input field, click Reset.
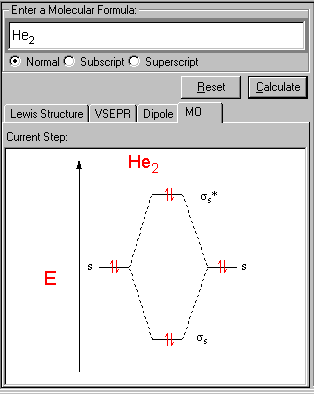
The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = 1/2 x (2-2)=0.