Kinetic-Molecular Theory is to model the properties of an ideal gas. The postulate of the theory is that gases consist of particles, and have the following properties:
1. The volume of the individual particles is negligible.
2. The particles are in constant motion.
3. No forces exert between the particles.
4. The average kinetic energy of a collection of gas particles is assumed to be directly proportional to the Kelvin temperature of the gas.
According to the Kinetic Molecular Theory, the Average Kinetic energy, (KE)avg can be represented as:
(KE)avg = (3/2) RT
and the Root Mean Square Velocity, Urms, is:
Urms = square root (3RT/MW)
Where:
R is ideal gas constant, R = 8.3145 J/K mol;
T, Kelvin temperature; and
MW, molecular weight.
The force exerted per unit area of surface, typical pressure units are ATM, mmHg and kPa.
The measurement of space taken by a substance, it is length cubed, typical units are L, mL and m3.
A measure of the average kinetic energy of the particles in a sample of matter, expressed in terms of units or degrees designated on a standard scale. Typical units are K, F and C.
The mass of the object divided by its volume. Typical units are g/mL and kg/m3.
The formula weight of a compound is the sum of all the atomic weights of the elements present in the formula of the compound. Some text also refers it to formula mass. Typical unit is g/mol.
Mass is the amount of a substance in grams, also called weight.
The constant that appears in the ideal gas equation (PV=nRT). It is usually expressed as 0.08206 L x atm/K x mol or 8.314 J/K x mol.
The number of moles of solute dissolved in one kilogram of solvent.
The number of moles of solute in one liter of solution.
This module is to compute the Average Kinetic Energy by given the temperature or Root Mean Square Velocity by known temperature and the molecular weight of the gas molecule.
In addition to the problem solving module, the "Show Work" is also displayed along with the solution to illustrate the step-by-step guide in how your problem has been solved.
This is two- step process, enter the known data and press Calculate to output the unknowns for each process
1. Select Kinetic-Molecular Theory of Gases link from the front page or Kinetic tab from the Gases module.
2. To calculate the KE, In the Temperature field, enter a temperature with a proper significant figure. Select the unit associated with the input. Click Calculate.
3. To Calculate the Root-Mean-Square Velocity, enter the formula of the molecule and the temperature with the unit. Click Calculate.
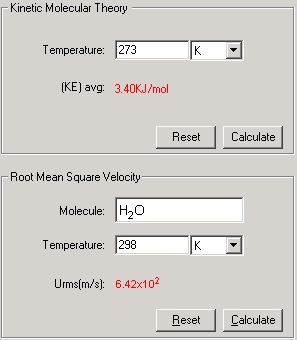
4. The Show Work area on the right shows you step-by-step how your problem has been solved.
To start a new problem, click Reset. All Input fields will be cleared. Follow Step 1-3 again.
In this program, entering text for molecular formula and chemical equation is rather straight-forward with relatively free style. The program follows chemist's rules, as appeared in chemistry text books. Here are the detailed instruction for entering a formula:
Formula is input as is, element symbols should start with capital letter and follow by low-case letter if dual-letter elements (e.g. for sodium, Na is correct, but NA, nA or na is not acceptable).
Subscription or superscription is entered by typing the upper(subscript)/lower(superscript) arrow keys on the keyboard.(e.g. for SO42-, the keystroke sequence is <S>-<O>-<Down-Arrow>-<4>-<Up-Arrow>-<2>-<Up-Arrow>-<->.