The concentration of a solution is often expressed as the percentage of solute in the total amount of solution. For the extremely dilute solutions the concentration unit parts per million (ppm) is often used. Since the amounts of solute and solution present can be stated in terms of either mass or volume, different types of percent and ppm exist:
concentration = mass solute / mass solution
concentration in mass %= (mass solute / mass solution) x 100
concentration in ppm (m/m) = (mass solute / mass solution) x 106
1. Calculate concentration by inputting the solute and solution amounts in the above formulae:
concentration = solute / solution
The mass of solutions is sum of the mass of solute and the mass of solvent:
solution = solute + solvent
2. Knowing the concentration and one of three: solute, solvent or solution, calculate the other two:
a. knowing solute
solution = solute / concentration
solvent = solution - solute
b. knowing solvent
solute = concentration x solvent / (1 - concentration)
solution = solute + solvent
c. knowing solution
solute = concentration x solution
solvent = solution - solute
This is one step process, enter the known data and press Calculate to output the unknowns.
1. Select Percent by Mass link from the front page or Percent by Mass tab from the Solution module. The Input and Output screen appears.
2. In the Input area, enter the two known quantities with a proper significant figure. Select the units associated with the input.
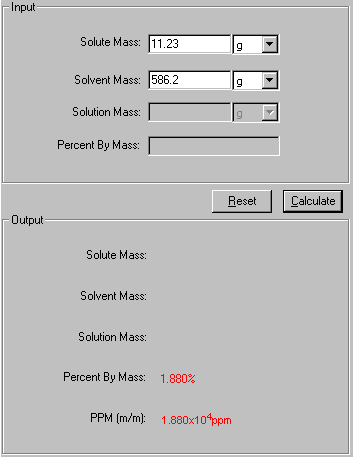
3. Click Calculate to output the answer.
4. The Show Work area on the right shows you step-by-step how your problem has been solved.
To start a new problem, click Reset. All Input fields will be cleared. Follow Step 1-3 again.